Provides speedy solutions to pharmaceutical affairs consultations in the medical device industry! Consultation service by e-mail "Help Desk for Pharmaceutical Affairs" opened on May 15.
A new corporate service for the medical device industry that provides easy consultation via e-mail on questions related to the Pharmaceutical Affairs Law and related standards. New service for corporate customers to easily consult with us via e-mail
株式会社東京メディカルデバイス・エージェンシー
Tokyo Medical Device Agency, Inc. (Head office: Machida City, Tokyo; President: Mikio Maeda) has established a new corporate service, "Help Desk for Pharmaceutical Affairs," to solve the problem of insufficient human resources and to improve operational efficiency in order to promptly respond to interpretations and questions regarding the "Act on Quality, Efficacy and Safety Assurance of Pharmaceuticals and Medical Devices" (hereinafter referred to as "Pharmaceutical Affairs Law") and related standards in operations related to pharmaceutical affairs and QMS (quality management system) of medical devices. The "Pharmaceutical Affairs Help Desk" is a new service for corporate clients that solves the problem of a shortage of human resources and improves operational efficiency. The "Pharmaceutical Affairs Help Desk" is a service that helps improve the knowledge of pharmaceutical affairs and quality assurance staff and enables simple consultation via e-mail. This service will be available to medical device manufacturers and distributors in Japan from May 15, 2025.

Help Desk for Pharmaceutical Affairs
Compliance with the "Pharmaceutical Affairs Law" in Japan is important for manufacturers and producers handling medical devices to maintain and manage them with proper understanding. For this purpose, it is essential to have personnel with specialized knowledge and the ability to promote the business. In recent years, however, the shortage of personnel with such expertise has become more pronounced, and outsourcing has become increasingly important.
In such an environment, companies are facing issues such as the lack of an in-house team system that can be consulted, and the inability to resolve questions in the midst of busy daily operations. As one of the solutions to these issues, we have established a new service for corporate customers, the "Pharmaceutical Affairs Help Desk.
The "Pharmaceutical Help Desk" was established to meet the demand for professional support, and offers three plans to meet the diverse needs of our customers. This provides a cost-effective option while eliminating waste.
The "Pharmaceutical Helpdesk" is a service that provides reasonably priced primary responses within 24 hours (excluding Saturdays, Sundays, and holidays) in principle to consultations submitted through a dedicated e-mail address. The service is characterized by the fact that consultation matters are handled by staff with extensive experience in pharmaceutical affairs and quality assurance operations at medical device manufacturers and distributors, as well as at certification organizations.
This is expected to bring the following benefits to our clients
1) Reasonably priced pharmaceutical consultation
2) Smooth resolution of "Pharmaceutical Affairs Law" and "related standards" questions
3) Compensate for human resource shortages and improve the ability to promote business operations.
4) Support for improving the knowledge of pharmaceutical affairs and quality assurance staff
Details of the "Pharmaceutical Affairs Help Desk" service can be found on the Tokyo Medical Device Agency, Inc. website (URL: https://t-mda.com/helpdesk ). You can also use the inquiry form to facilitate the contracting process. Fees are listed in the Appendix. The goal for the "Pharmaceutical Affairs Help Desk" is to have 100 new contracts by the end of 2026.
Overview of the "Pharmaceutical Affairs Helpdesk
Service name: "Pharmaceutical Affairs Helpdesk
Service contents and fees: As shown in the table below.
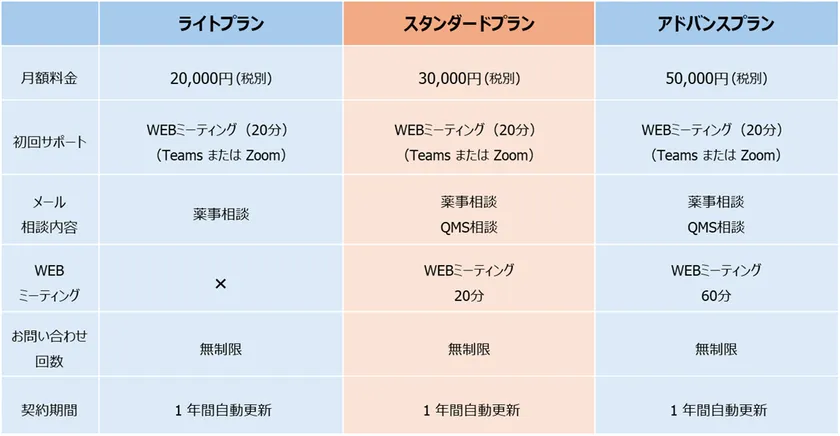
Service Description and Fees
As a commemoration of the opening of the "Pharmaceutical Affairs Help Desk," a 6-month trial contract is available!
<Pharmaceutical Affairs Consultation
General consultation for registered certification bodies, pharmaceutical applications (approval, certification, notification), judgment on the type of change (partial change, minor change, etc.) for previously approved/certified items, QMS conformity surveys, Pharmaceutical Affairs Law surveillance audits, five-yearly surveys, interpretation of pharmaceutical affairs-related notices and administrative communications, PMDA consultation services, registered certification body consultation services, business licensing and registration, consultation on the ministerial ordinances for medical devices, etc. Consultation on the Ministerial Ordinance of the Ministry of Health, Labour and Welfare, and other matters related to pharmaceutical affairs, etc.
<QMS Consultation
Questions regarding interpretation of ISO13485 certification bodies in general, QMS ministerial ordinances/ISO13485 requirements, QMS operation, internal audits, ISO13485 audits, QMS-related notifications, administrative communications, etc., QMS miscellaneous consultation, etc.
URL of product/service site: https://t-mda.com/helpdesk
Service start date: May 15, 2025
Company Profile
Company name: Tokyo Medical Device Agency, Inc.
Representative : Mikio Maeda
Establishment : January 2025
Location : 8-17-2 Naruse, Machida-shi, Tokyo 194-0044
HP : https://t-mda.com/
Description of Business: QMS (Quality Management System) for medical devices,
Consulting services for QMS (Quality Management System), approval and certification applications for medical devices,
Consulting services for medical device business
- Category:
- Services