Low-cost access to Japanese and U.S. regulatory databases Addition of U.S. FDA to High Precision RAG "STiV" Regulatory Information Collection Service
株式会社ファンリード
Fun Reed Corporation (headquartered in Toshima-ku, Tokyo; Keiichi Kobayashi, President & CEO; hereinafter "Fanreed") has expanded its service for collecting pharmaceutical affairs information on behalf of its generation AI knowledge management system "STiV". In addition to the existing information provided by the Japanese Ministry of Health, Labour and Welfare (MHLW), etc., STiV also includes guidance and other information provided by the U.S. Food and Drug Administration (FDA), and performs daily crawling and updating. This enables the use of Japanese and U.S. regulatory information at low cost.
STiV was developed in response to the needs of pharmaceutical manufacturing companies within the Taiyo Holdings Group, to which Fanreed belongs, and has undergone numerous upgrades in pursuit of greater operational efficiency for pharmaceutical companies. The number of companies in the pharmaceutical industry that have adopted STiV has more than tripled compared to last year, as it supports the streamlining of quality assurance and pharmaceutical affairs operations and the transfer of know-how.
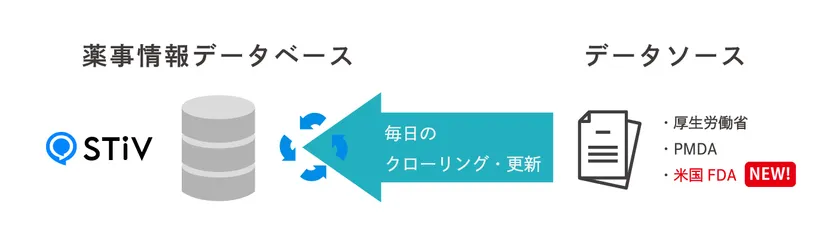
Image of Japan-US Regulatory Affairs Information Collection Agency Service
Main Features of the Regulatory Affairs Information Collection Service
As a pharmaceutical information database, it currently contains more than 50,000 files and is constantly updated with the latest information through daily crawling. The collection targets of this service are as follows.
Japan (service available from May 2024)
Various information issued by the Ministry of Health, Labour and Welfare (MHLW) and the Pharmaceuticals and Medical Devices Agency (PMDA)
Notification documents
GxP Ministerial Ordinance
Japanese Pharmacopoeia
Guidelines, etc.
U.S.A. (Service will start in January 2025)
Various information issued by FDA (U.S. Food and Drug Administration)
Guidance and guidelines
Form 483 inspection reports, etc.
Through STiV, Fanreed will provide a platform that enables the pharmaceutical industry to utilize information appropriately and promptly, thereby helping to improve operational efficiency and ensure compliance.
Generation AI utilization platform "STiV" website
Top page: https://www.stiv.jp/
Function overview page: https://www.stiv.jp/technology/#pharmaceutical-data
Fun-Read Corporation Company Profile
Established in 2013, Fanreed is a consolidated subsidiary of TAIYO HOLDINGS CO.
President and Representative Director: Keiichi Kobayashi
Establishment: March 26, 2013
Location: Metropolitan Plaza Bldg. 15F, 1-11-1 Nishi-Ikebukuro, Toshima-ku, Tokyo
Company HP : https://www.funlead.co.jp/
- Category:
- Corporate Trends