Japan's First Medical Image Data Based on the Next Generation Medical Infrastructure Act ~Programs to support physicians' diagnosis and research and development of medical devices and pharmaceuticals, etc. Supporting research and development of medical devices, pharmaceuticals, etc.
PSP Corporation
PSP Corporation (headquartered in Minato-ku, Tokyo; Yoshihisa Yoda, President; hereinafter "PSP"), together with the Life Data Initiative (hereinafter "LDI") and NTT DATA Corporation (hereinafter "NTT DATA"), will start providing medical image data such as X-ray images as anonymous processed medical information in October 2024, based on the Next Generation Medical Infrastructure Act (Note 1). (hereinafter referred to as "NTT Data") will start providing medical image data such as X-ray images as anonymized processed medical information from October 2024. Inquiries regarding this matter will be accepted from June 2024. This will be the first time in Japan that medical image data based on the Next Generation Medical Infrastructure Act has been provided.
In recent years, research and development of programmed medical devices (Note 2) that use AI to assist doctors in diagnosis has been conducted worldwide, but in Japan, the lack of medical image data necessary for research and development and the complexity of procedures for acquiring such data have been issues. In addition, the use of medical images has required a high level of anonymity processing technology that takes confidentiality into consideration. In response to these issues, PSP acquires medical image data, LDI and NTT Data anonymously process the data, and provide it to companies that research and develop programmed medical devices, etc., thereby creating an environment in which medical image data can be easily utilized.
Through this initiative, LDI and NTT Data will support research and development of programmed medical devices, etc., and contribute to the Japanese medical industry through earlier and more precise diagnosis and reforms in the way doctors work by reducing their working hours.
Background and History
Medical image data is image data obtained from imaging tests (X-ray, CT, MRI, ultrasonography, endoscopy, pathology, etc.) performed in the medical field, and is used for disease detection, diagnosis, and determination of treatment effects.
In the past, imaging tests were performed by specialized physicians who directly checked the medical image data to make a diagnosis. In recent years, however, research, development, and widespread use of programmed medical devices that support physicians' diagnosis, such as those that analyze medical image data using AI and other technologies, automatically detect abnormalities, and notify physicians of them, has been progressing worldwide. These devices are expected to support higher-precision diagnosis and contribute to reforming the work styles of busy physicians.
However, while Japan has one of the world's highest penetration rates for medical devices that perform imaging tests such as MRI and X-ray examinations, the number of programmed medical devices developed and approved is very low compared to Europe and the United States. One of the reasons for this is the high hurdle in acquiring medical image data for study and validation, which is necessary for research and development. Research and development of programmed medical devices that support image examination requires the use of a considerable amount of medical image data to improve the accuracy of AI analysis (Figure 1). Since medical image data constitutes sensitive personal information (Note 3) under the Personal Information Protection Law, it must be acquired with the cooperation and approval of medical institutions and with the consent of the individual concerned, in principle. The complexity of this procedure has become an issue, and a more convenient means of acquiring medical image data has been sought. In addition, because the data is personal information requiring special consideration, a high level of anonymity processing technology that takes confidentiality into consideration is also required.
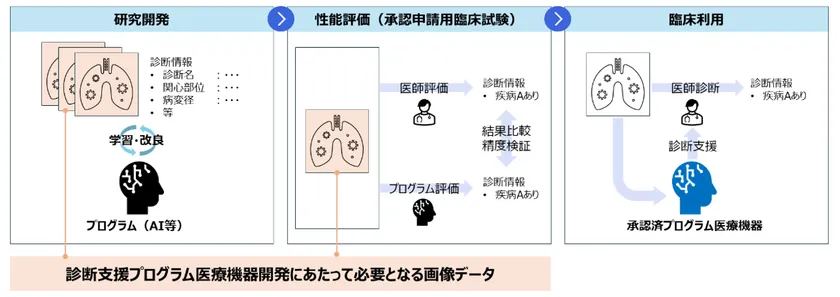
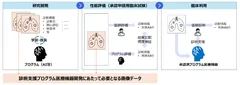
Figure 1: Flow chart of medical device development and required data for diagnosis support program
Overview of Medical Image Data Provision
LDI and NTT DATA will start providing medical image data from October 2024, based on the Next Generation Medical Infrastructure Act. Inquiries regarding this matter will be accepted from June. In March 2024, an application for the handling of medical image data was submitted to and accepted by the competent ministry (Note 4) of the Next Generation Medical Infrastructure Act.
The two companies have been providing electronic medical records, insurance claim data, and DPC survey data (Millennium Medical Record Secondary Usage Service (Note 6)) as real world data (Note 5) for medical institutions, and now medical image data will be newly added. This will create an environment where medical image data can be easily utilized. This is the first time in Japan that medical image data has been provided in accordance with the Next Generation Medical Infrastructure Act.
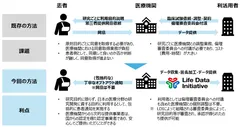
The provision of medical image data will be carried out in collaboration with LDI and NTT DATA, which are authorized providers (Note 7) under the Next Generation Medical Infrastructure Act, and PSP, a leading cloud-based medical image management system (Note 8). PSP will collect medical image data owned by medical institutions or academic societies, and link this data to LDI and NTT Data, which will process the data anonymously in accordance with the Next Generation Medical Infrastructure Act, including masking of personal information in the images and reproduction of facial expressions by 3D reconstruction in CT and MRI examinations, The data will be provided to businesses, academia, etc. that wish to use it for the purpose of research and development in the medical field. (Figure 2)
Data users can obtain medical image data from LDI without the need to obtain individual patient consent, and by implementing appropriate opt-out (Note 9) from medical institutions, etc. to patients. As LDI is an accredited entity under the Next Generation Medical Infrastructure Act, medical institutions and patients can feel secure in providing their data. (Figure 3)
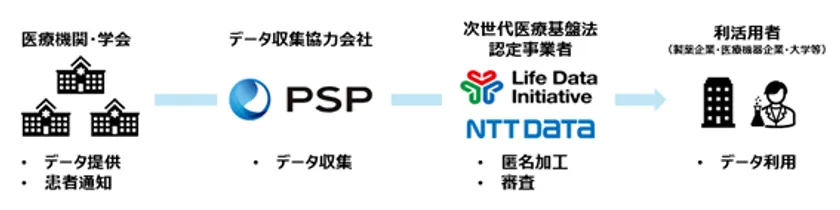
Figure 2: Image of the initiative
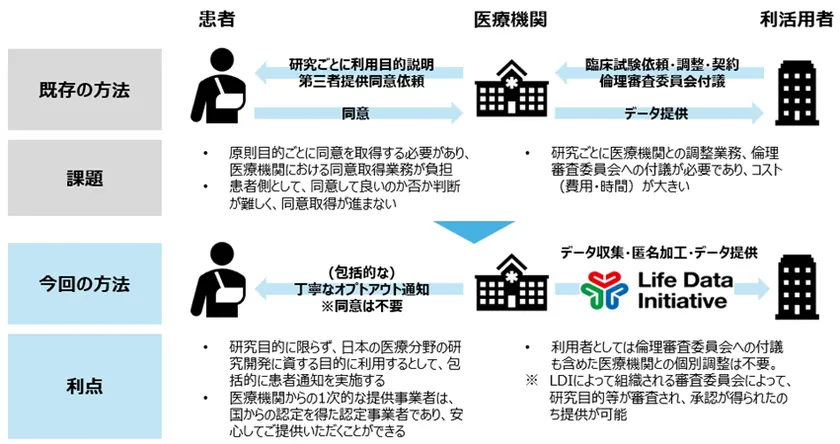
Figure 3: Issues to date and benefits of this initiative
Use Cases
The launch of this service will contribute to the promotion of programmed medical device development, etc., as follows
(1) Utilization in the development of cancer detection support programs
One of the challenges in the medical field is that it is difficult to detect some types of cancer, and if cancer is detected at an advanced stage, it is difficult to cure it completely. In response to this, if a programmed medical device could be developed that detects and indicates possible cancerous lesions, it would provide an opportunity for doctors to examine the lesions at an early stage. The medical image data provided in this project may be utilized as data necessary for the development of such a program. 2.
(2) Application to the development of programs to support the determination of disease severity
One of the issues in the medical field is that the severity of illness is sometimes evaluated based on the patient's subjective opinion, and objective assessment of severity may not be possible. In such cases, it is difficult to flexibly select or change treatment according to the severity of the disease. In contrast, if a programmed medical device that indicates numerical values related to the severity of the disease is developed, it will be possible to select initial treatment based on the relevant values and to flexibly search for a suitable treatment for the patient, focusing on changes after a specific treatment. The medical image data provided in this study may be utilized as data necessary for the development of such a program.
Future plans
In the future, it is expected that the creation of more accurate real-world evidence and the research and development of more accurate diagnostic support program medical devices, etc. will be promoted by combining existing anonymous processed medical information such as electronic medical records and insurance claim data with medical image data. Through this initiative, we will support the research and development of diagnostic support program medical devices, etc. and pharmaceuticals, and contribute to the Japanese medical industry through earlier and more precise diagnosis and reforms in work styles by reducing doctors' working hours.
[Notes
(Note 1) Official name: "Act on Anonymously Processed Medical Information and Pseudonymously Processed Medical Information for the Purpose of Contributing to Research and Development in the Medical Field. The law is intended to process personal information of citizens and patients so that individuals cannot be identified, and to use it for research and development of new drugs and treatments.
(Note 2) Medical device programs that assist physicians in diagnosis. If it falls under the category of medical devices, it is subject to regulation and requires approval and certification.
(Note 3) Personal information that includes the person's race, creed, social status, medical history, criminal record, the fact that the person has been harmed by a crime, and other descriptions specified by a Cabinet Order as requiring special consideration in handling to prevent unjust discrimination, prejudice, or other disadvantages to the person.
(Note 4) Cabinet Office, Ministry of Health, Labor and Welfare, Ministry of Economy, Trade and Industry, and Ministry of Education, Culture, Sports, Science and Technology.
(Note 5) Medical data recorded from actual medical treatment at medical institutions.
(Note 6) The Millennium Medical Record is an initiative based on the "Act on Anonymously Processed Medical Information for the Purpose of Contributing to Research and Development in the Medical Field" (Next Generation Medical Infrastructure Act) to provide medical information obtained from medical institutions, etc. to data users through statistical processing or anonymized processing to help in advanced research and development related to health and medicine and in the creation of new industries. This is an initiative to provide medical information obtained from medical institutions, etc. to data users through statistical processing or anonymous processing, and to use the information for advanced research and development related to health and medical care and for creating new industries.
(Note 7) LDI: Authorized anonymous processor of medical information; NTT Data: Authorized entrusted operator of medical information, etc.
(Note 8) A system for storing and viewing the results of imaging tests (image data) in hospitals.
(Note 9) The individual or his/her bereaved family does not request that the system be stopped after being notified in writing at the time of the first basic medical examination at a medical institution, etc.
About Life Data Initiative, Inc.
(Life Data Initiative, Inc. has been certified by the government as an accredited provider under the Next Generation Medical Infrastructure Act, and promotes the business of utilizing medical information by collecting medical information on all aspects of health and medical care, and then developing services for users and participating facilities, thereby contributing to the safe and effective utilization and research and development of medical and pharmaceutical products. NTT DATA aims to realize the provision of medical care using the world's highest level of technology that contributes to the safe and effective utilization and research and development of medical and pharmaceutical products.
About NTT DATA Corporation
NTT DATA Corporation provides IT services in more than 50 countries around the world, aiming to create a prosperous and harmonious society. NTT DATA provides a wide range of services from consulting to system development and system operation, looking to the future together with its customers to transform business and solve social issues through the use of digital technology.
About PSP Corporation
PSP is a healthcare IT solutions company that provides PACS (medical image management system), RIS (radiology information system), and PHR (personal health record), including cloud-based systems that can safely store, utilize, and share medical information. Based on our company's mission statement, "Putting medical information in everyone's hands and into the future," we are committed to providing medical information solutions to our customers. Our goal is to contribute to the creation of a sustainable healthcare environment by building a healthcare information infrastructure and providing highly convenient medical services that share the "aspirations" of patients, healthcare professionals, families, and local communities.
- Category:
- Corporate Trends